Superabrasives remove material in a unique way, due to their super hardness. The whole process is vaguely similar to the milling operation. The best way to explain this is by describing the basic mechanism of work being performed by a single superabrasive grit particle.
Looking at a single grit particle during one pass through the workpiece, we see several phases of engagement. In order to penetrate the workpiece surface and start the cutting process, the grit particle must first overcome the forces from plastic deformation at the surface of the workpiece. Sharp grit will start cutting soon after a small penetration has been made. Dull grit must penetrate the surface much deeper before it starts cutting. Deeper penetration requires higher force, which creates more heat going into the workpiece.
Each engaged grit particle must frequently micro-chip to resharpen itself when it becomes dull. For this reason the grit particle should be strong enough to cut the workpiece, but when it becomes dull, it should micro-chip. The depth of the grit particle penetration determines the performance of the grinding. Optimal penetration makes the grit particle micro-chip gradually. If the grit particle is overly strong, then it will not micro-chip. Instead it will macro-fracture, or if the particle bonding force is weak, the grit will peel off or pull out. Micro-chipping of grit particles, together with some macro-fracture and eventual peeling of the grains throughout the wheel life, progressively increase the topographical density of active grains, which actually improves the finish of the ground surface.
Most often both CBN and diamond grits are single crystals. There is the possibility of using polycrystalline CBN grit, for example (GE 550 series), which is easier to micro-chip and creates sharp edges.
Let us now focus our attention on high-speed grinding which takes advantage of the easier forming of chips. Cutting forces decrease substantially during increased cutting speeds because a higher temperature at the cutting zone decreases the rapture point of the workpiece material. If correctly applied, increased feed speed or infeed will reduce grinding time, while the cutting forces will remain almost constant.
The chip size gets smaller at higher cutting speeds, which means that less thermal energy goes into the workpiece and more thermal energy is taken out by the chips. Thermal performance is improved due to the extremely brief length of time when the abrasive grit is in contact with the workpiece. Friction heat initially spreads out over the workpiece surface before sinking into the workpiece. The advantage of high-speed grinding is that the next chip that is cut out takes the previously generated surface heat away.
It should be noted that for successful high speed grinding it is crucial to match the coolant velocity directly into the grinding zone. In addition, there are many more necessary requirements for this relatively new process. These include substantially increased demand for the power to the grinding spindle, and also much higher overall stiffness of the grinding machine.
High-speed grinding is practical for roughing operations, where heavy stock removal is desirable. High-speed grinding for finishing operations may not be practical due to the minimal stock left for finishing, which limits the loading condition necessary for the proper micro-chipping of the grains crucial to maintain the sharp condition of the grinding wheel. Safe operation of plated CBN wheels has been tested up to 500 m/s. The high thermal resistance of CBN makes the high-speed grinding process possible.
So let’s examine the thermal and chemical considerations of grinding with superabrasive grit particles a bit closer. CBN has higher thermal resistance than diamond. As a matter of fact, at temperatures exceeding 800ƒC, CBN is harder than diamond and is able to maintain its cutting edge well up to 1000ƒC. In addition, the thermal conductivity of CBN is four and half times better than copper–in close competition with diamond.
Thermal energy partition, which is a fraction of the grinding energy transported as heat into the workpiece at the grinding zone for CBN plated wheels, is below 10 percent. Just for comparison, Al-oxide grinding wheels have an energy partition value of around 70 percent, and for vitrified CBN wheels it is approximately 20 percent. This means that more than 90 percent of the grinding heat is removed from the grinding zone with the CBN plated wheel grinding process.
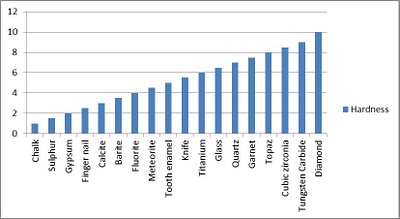
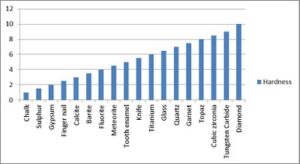
Strength and resistance to the thermal and chemical attack while maintaining sharp cutting edges during use makes CBN the superior choice compared to conventional abrasives such as silicon-carbide and aluminum-oxide. In addition, high thermal stability makes CBN the superabrasive of choice for the grinding of all varieties of steel alloys–an area where diamond abrasives are not normally employed.
Even with a melting point of 4,000ƒC, which is the highest of all materials, diamond has substantial thermal limitations in many grinding applications:
- Diamond will oxidize above 600ƒC into carbon-dioxide.
- Diamond has a tendency to react with the iron group metals (iron, cobalt, and nickel and its alloys) already at a temperature above 700ƒC. These metals are soluble in carbon, and diamond is just another form of carbon. Synthetic diamond is produced by catalytic conversion under high pressure, and metal inclusions of catalytic metal contaminate each crystal. Metal inclusions will convert the diamond back into a graphite type of carbon at temperatures above 700ƒC. Back-converted graphite expands inside the diamond crystal and creates microscopic cracks, which cause so-called “thermal weakening” of synthetic diamond.
- Diamond reacts with carbides (titanium-carbide, tungsten-carbide, and zirconium-carbide) already at a temperature above 700ƒC by converting back to amorphous carbon.
- Diamond will react with carbide formers (titanium and vanadium) above 800ƒC to convert into carbides.
- Diamond could be dissolved in transition elements of iron group metals or their alloys above 900ƒC
In light of this, diamond cannot be used to grind steel or titanium alloys if the temperature exceeds 700ƒC. This makes it impossible for synthetic diamond to be used for the most common grinding applications, such as the grinding of hardened steel gears. This high rate of wear would make diamond uneconomical in comparison to CBN, or even other conventional abrasives.The biggest advantage of diamond is its high thermal conductivity (five times greater than copper). This high thermal conductivity allows diamond to transfer most of the heat away from the cutting edge and dissipate the thermal energy through the grinding wheel body into the grinding coolant, which hits the grinding wheel at a high flow rate. This makes diamond the top-choice superabrasive for any material which is not soluble in carbon (non-metal materials). These include non-ferrous metal grinding (copper or aluminum alloys); cermets of ceramic (cemented tungsten-carbide, metal matrix composite Al-Si-C); and glass, silicon, granite, or marble.While the crystal structure of diamond and CBN is very similar, there is one difference: the carbon atoms of the diamond crystal structure have four valence electrons. Tetrahedral bonds inside the diamond crystal structure tie all four electrons. However, on the surface, only two or a maximum of three electrons can create a permanent bond. The remaining one or two electrons from each atom on the surface have a tendency to react with iron, cobalt, nickel, or oxygen at high temperature.CBN has the same crystal structure as diamond, except boron-nitrogen bonds replace the carbon-carbon bonds in diamond. This makes CBN less reactive at high temperatures. Boron atoms in CBN crystal have only three valence electrons. Nitrogen atoms in CBN crystal have five valence electrons, but two of five electrons will form a stable pair on the surface, leaving three valence electrons for bonding with boron. Therefore the surface of CBN contains a reduced amount of free “dangling” electrons, which makes CBN more stable at high temperature. CBN also doesn’t contain the carbon, so it doesn’t react with a metal group the way that diamond does.Abrasion resistance and hardness are very important physical properties of an abrasive. Both diamond and CBN are almost equal, with about four times the abrasion resistance of Al-oxide. However, while CBN is substantially harder than other conventional abrasives, it has only 64 percent the hardness of diamond–45,000 N/mm2 Knoop, as compared to diamond’s 70,000 N/mm2 Knoop. (Hardness of Al-oxide is 20,000 N/mm2 Knoop.)Finally we will discuss the choice of fluids for grinding with superabrasives. Grinding fluids perform several important functions during the grinding process, including chip removal from the grinding zone, heat removal, and protection from chemical reactions during the grinding interaction. Oil-based grinding fluids provide the best performance when employing CBN grit. Water-based grinding fluids are not suitable for CBN grinding applications for a number of reasons. Low vaporization temperatures (water-based soluble oils vaporize at 130ƒC) limit the fluid’s lubricity, and high-temperature steam promotes continuous oxidation at the surface of the CBN crystal.The exposed surface of CBN crystals will oxidize even in oil-based grinding fluids at high temperature. However, this oxidizing is terminated as soon as a protective layer of (B2O3) oxide covers the exposed surface. Unfortunately this (B2O3) oxide is soluble in steam of higher temperatures, which means that water-based grinding fluids under a high temperature would promote further oxidation. This oxidation will result in an accelerated breakdown of the CBN particles and shortening of the grinding wheel life.Oil-based grinding fluid provides good lubrication, but poor cooling. The use of oil reduces the friction in the grinding zone and will result in lower grinding power. The boiling temperature for oil coolant is 3OOƒC, which decreases the occurrence of the oil film boiling. Oil-based coolant is excellent regarding the machine component maintenance, yet there are many environmental dangers. Also, filterability is much more difficult. The need for lubricity is not as crucial when grinding with diamond as it is when grinding with the CBN.
Synthetic diamond is manufactured by heating and compressing graphite to 4,000ƒC @ 12,000 N/mm2, shock conversion of graphite at 30,000 N/mm2 @ 500ƒC or static conversion from graphite at 13,000 N/mm2 @ 3000ƒC. Diamond was first synthesized by the physicist Rrik Lundblad at ASEA Company in Stockholm, followed by Francis Bundy of the General Electric Company in 1954.
CBN is not present in nature. It is produced by the catalytic synthesis of hexagonal boron-nitride under static high pressure and high temperature conditions. CBN was first synthesized by Robert Wentorf (also of GE) in 1957.
The speed of this process is substantially slower than the similar process for synthesized diamond. Thin-film CBN crystals can also be grown from the vapor state by ion beam or plasma deposition. Pure CBN is transparent or slightly amber. Magnesium or carbon will create black CBN, and silicon will make CBN red.
Both diamond and CBN are excellent choices in progressive grinding programs. We all look forward to a better, harder-than-diamond, novel synthetic super-hard abrasive such as carbon-nitride (C3N4), which was theoretically proposed by James C. Sung in 1984.
Manmade diamond, and synthetic CBN were developed by the successful synthesis of light elements such as boron, carbon, and nitrogen. Extremely strong and short covalent bonds of these elements can form very tight, three-dimensional networks with extreme resistance to external forces. Practical methods to produce these materials on a large industrial scale still do not exist. Until that time comes, we continue to push the limits with better and faster grinding systems.
 Contact Us
Contact Us  Phone
Phone